Isotoma anglicana/caerula and Isotoma viridis can only be distinguished by examining the number of teeth on the dorsal apical edge of the manubrium (Isotoma anglicana has two teeth on each side, Isotoma viridis has one). It is not essential to clear the specimen to do this, but depigmentation usually makes the teeth much easier to see.
Isotoma anglicana/caerula, pigmented specimens:
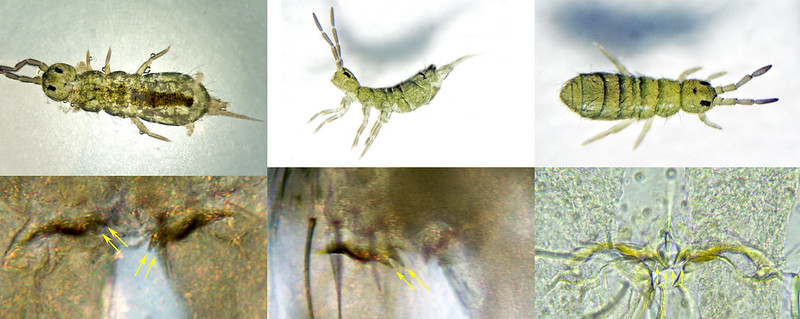
Isotoma anglicana/caerula, cleared specimen:
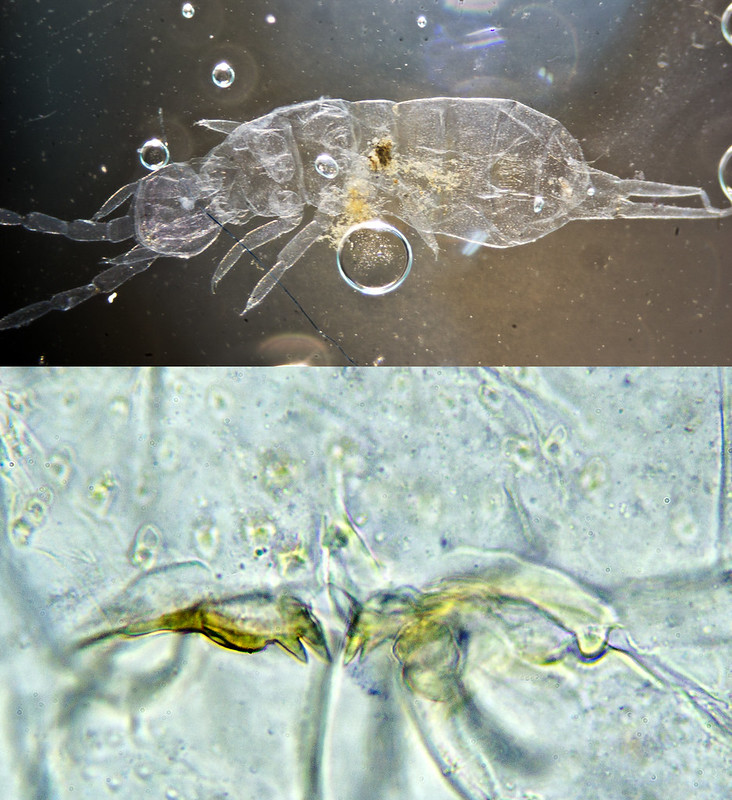
Clearing Method:
Make a solution of 10% sodium hydroxide (NaOH) (careful, this is caustic).
Transfer the specimen to a small, sealed tube containing approximately 0.1 mL of the alkaline solution.
Float the tube on hot water for 5-15 minutes (you may need to experiment to find the optimum time).
Transfer the specimen into a drop of plain water on a microscope slide and cover with a glass coverslip.
Examine under the microscope.
The first time you do this it may seem off-putting or too complex to bother with, but if you have a supply of specimens to practice on, it soon becomes fairly easy, although if you're as ham-fisted as me you will ruin the odd specimen, so it helps to have more than one! On a number of occasions I have accidentally pinged a specimen across the room while transferring it from tube to slide - good luck finding it on the carpet!
No comments:
Post a Comment
Comments welcome, I will respond as soon as I can.
Note: only a member of this blog may post a comment.